Quality B cleanrooms are utilized for aseptic planning, filling, and compounding procedures. They're such as ISO Class 5 cleanrooms at rest and ISO Class 7 cleanrooms in operation.
Planning of elements and most merchandise ought to be performed not less than in a very Grade D cleanroom. Still, some items with large or uncommon risks of microbial contamination must be geared up inside of a Grade C area.
Definition & analytics: Definition of exam and measurement conditions, progress of someone measurement program, testing of the material sample
The checking of the Grade C area should be applied in line with good quality hazard management ideas, the systematic process for evaluating, managing, speaking, and reviewing dangers to the quality of a medicinal merchandise all through its lifespan.
Know Much more › What is the highest quality of cleanse home? › ISO one could be the “cleanest” class and ISO 9 may be the “dirtiest” class. Even though It truly is classified as the “dirtiest” class, the ISO 9 cleanse space environment is cleaner than a regular space.
Visualization scientific studies enable validate the design and performance of the cleanroom’s ventilation procedure, guaranteeing that the air moves inside the meant paths to maintain cleanliness.
The main difference between OTC and health-related or pharmaceutical-grade skincare merchandise is the fact that one can only be prescribed by a clinical Skilled.
There may be NMFC codes that class dependant on how an product is packaged, its value, or every other solution characteristic. The one way to be aware of needless to say would be to Get the LTL transport specialist that may help you lookup your item in the NMFC databases.
Continue reading › Is D deemed failing? › A letter quality of the D is technically thought of passing as it not a failure. A D is any share concerning sixty-69%, whereas a failure happens underneath 60%. Despite the fact that a D is a passing grade, It really is hardly passing.
(a) To get to the B, C, and D air grades, the volume of air adjustments really should be associated with the scale from the place and also the devices and personnel present inside the area. The air process should be supplied with ideal filters which include HEPA for grades A, B, and C.
They are as follows: Qualitative Investigation: This process is utilized for the identification of the chemical click here compounds. Quantitative Investigation: This method is utilized for the perseverance of the quantity of the sample. ...
The Quality D atmosphere is usually a history zone, depending on how your cleanroom is designed. Here is the least clean area of your GMP necessities for sterile products.
Steady control of these elements is essential for the stability of goods and also the prevention of conditions that could promote microbial development or compromise item excellent.
Layout qualification verifies the cleanroom design and style can meet up with all regulatory and approach necessities. It makes sure that the get more info cleanroom is intended to give the required amount of Handle and cleanliness.
 Emilio Estevez Then & Now!
Emilio Estevez Then & Now!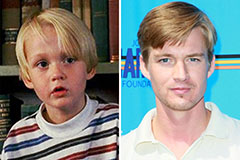 Mason Gamble Then & Now!
Mason Gamble Then & Now!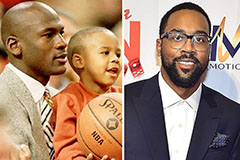 Marcus Jordan Then & Now!
Marcus Jordan Then & Now! Bernadette Peters Then & Now!
Bernadette Peters Then & Now! Jaclyn Smith Then & Now!
Jaclyn Smith Then & Now!